Hydrogen Molecule in the Light of the de Broglie Theory
Pavle I. Premović
Laboratory for Geochemistry,
Cosmochemistry&Astrochemistry,
University of Niš, pavleipremovic@yahoo.com, Niš, Serbia
The de Broglie wave equation for a massive particle in motion is given in the following form
λ = h/mυ ... (1)
where λ is the
wavelength, h (= 6.63 × 1034 J sec)[1] is Planck's constant and m
is the mass of a particle, moving at a speed υ.
If H2 travels with a non-relativistic speed υ its wavelength is then
λ(H 2) = h/m(H2)υ ... (2)
where m(H2) is the rest mass of H2.
The mass of the electron is negligible compared to the mass of proton m(H+) then the mass of H2 m(H2) ≈ 2m(H+) ≈ 3.34 × 10-27 kg. The most probable/average speed of H2 which occupies 22.1 dm3 at STP is υmp(H2) ≈ 1500 m sec-1. Using eqn. (1), a simple calculation shows that its average wavelength λ(H2) ≈ 1.3 ×10-10 m is about twice larger than the bond length (аbout 7.4 × 10-11 m) of H2.
The wavelength of each proton in this moving H2 is
λ(H+) = h/m(H+)υ.
Neglecting again the mass of the electron, we have m(H) = m(H+) or m(H+) = 1/2 m(H2). Plugging this last term into eqn. (3) and taking into account eqn. (2) we get
λ(H+) = 2λ(H2) ... (3).
Using this equation, a simple calculation shows that the average wavelength of the protons in H2 λ(H+) ≈ 2.6 × 10-10 m.[2] This is four times larger than the bond length of H2.
As we
pointed out before {1}, the superposition of the waves of these protons can
result in two types of interference depending on their phasing. If they are in
phase we deal with constructive interference. In this case, the resultant wave
would have twice large an amplitude as the proton but its wavelength would be the
same as the proton wavelength. If they are out of phase, we deal with the
destructive interference, the two proton waves would cancel each other so there
would be no resultant wave.
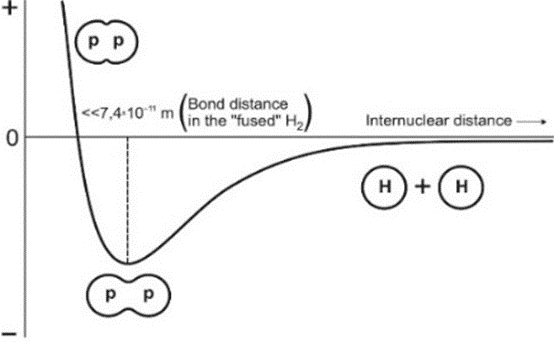
When the protons of H2 are so far apart (about 43500 proton diameters) one is dealing virtually with two completely separated nuclei. In contrast, de Broglie’s concept would imply that the protons do not exist separately in H2.[3] Having that in mind and the de Broglie eqn. (1), we speculate, with great hesitation, that the H2 nucleus is made of two proton nuclei partly “fused” forming the diproton nucleus, H22+ (or “p-p”). We also hypothesize that H22+ is dumbbell-shaped, Fig. 2.[4] Its two e = 1+ charges are separated from each other by an internuclear distance much smaller than 7.4 ×10-11m or << 7.4 × 10-11 m.[5] At this distance the potential energy of H2 with the partly “fused” protons is at a minimum, Fig. 1. This distance is the bond length of H2 with partly “fused” protons.
Fig.
1: Potential energy vs. internuclear distance between two partly “fused” diprotons
of H2.
Fig. 2: Highly exaggerated two-dimensional picture of the (dumbbell
shape) partly “fused” diproton nucleus of H2.
References
[1] To avoid confusion in further text, the SI units are given in italics.
[3] As far as we are aware, this claim has not been demonstrated experimentally.
[5] It is interesting to note that Holmlid and Zeiner-Gundersen {2} reported the existence of another species of H2 with bond length 2.3 × 10-12 m. However, even at this bond length, the protons are very far from each other: about 14,500 proton diameters.



.jpg)

No comments:
Post a Comment