Highly Exaggerated
Representations of the Hydrogen Atom and Molecule
in Elementary
Textbooks of Physics and Chemistry
Pavle I. Premović,
Laboratory for Geochemistry, Cosmochemistry&Astrochemistry,
University of Niš, pavleipremovic@yahoo.com, Niš, Serbia
In elementary
physics and chemistry textbooks, we find the following (or similar) illustration
of the Bohr model hydrogen atom at its ground state, Fig. 1. Proton (
Fig. 1: Bohr model of the hydrogen atom.
The value of the radius of this orbit is a physical constant
that is approximately equal to 5.3 × 10-11 m. The (charge)
radius of the proton is about 0.85 × 10-15 m.
Thus, the mean distance between electron and proton is equal to about 31000 proton
diameters. In other words, the representation of the Bohr hydrogen atom model
given in Fig. 1 (or similar) is highly exaggerated. As far as we are aware, this is not clearly emphasized in any of the
textbooks mentioned above.
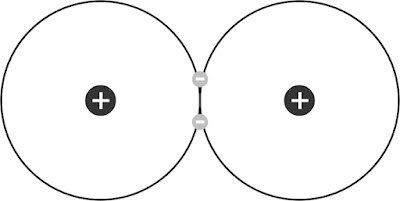
Fig. 2: Lewis structure of hydrogen molecule.
We encounter a
similar problem in these textbooks when they talk about hydrogen molecule. This
molecule is usually illustrated by the so-called Lewis (electron pair) structure
as in Fig. 2 (or similar). This structure is based on Bohr’s model of the hydrogen atom.
Each
hydrogen atom in this molecule contains one electron and one proton. These
atoms form a chemical (covalent) bond by sharing one electron each. The optimal internuclear distance between the two protons of
the hydrogen molecule is named the bond distance (or length).
This distance is equal to about 7.4 × 10-11 m or about 43500 proton
diameters apart. In other words, this or similar pictures of hydrogen molecules
are also highly exaggerated. As far as we are aware, this is not explicitly
indicated in elementary textbooks of physics and chemistry.
Fig. 3: Molecular orbital model of hydrogen molecule: overlapping 1s orbitals.
The molecular orbital approach is superior in describing the bond between two hydrogen atoms in the hydrogen molecule over the Lewis electron-pair approach. It is reasonable to say that the Lewis electron-pair bond in this molecule is equivalent to the molecular orbital description of a bonding molecular orbital occupied by a tightly paired of 1s electrons, Fig. 3. Thus, the exaggerated picture of the hydrogen molecule in textbooks of physics and chemistry, based on the theory of molecular orbitals, is similar to that of Lewis's picture in these books.




.jpg)

No comments:
Post a Comment